Quiz on osmosis and diffusion – Prepare to immerse yourselves in the captivating realm of osmosis and diffusion! This quiz will guide you through the intricate world of molecular movement, revealing the secrets behind the flow of life itself.
As we embark on this journey, we’ll unravel the mysteries of semipermeable membranes, concentration gradients, and the factors that govern the rates of osmosis and diffusion. Get ready to witness the dance of molecules as they traverse biological barriers, shaping the very essence of living systems.
Introduction
Osmosis and diffusion are fundamental processes that underpin numerous biological functions. Osmosis refers to the movement of water molecules across a selectively permeable membrane, driven by differences in water concentration. Diffusion, on the other hand, involves the movement of particles (such as molecules, ions, or gases) from an area of high concentration to an area of low concentration.
These processes play a vital role in maintaining cellular homeostasis, nutrient transport, waste removal, and overall physiological functions in living organisms.
Importance of Osmosis and Diffusion in Biological Systems
Osmosis is crucial for regulating cell volume and preventing cell damage. It ensures that cells maintain an appropriate water balance, preventing excessive swelling or shrinking. Diffusion facilitates the movement of essential substances, such as nutrients, oxygen, and waste products, across cell membranes and throughout the body.
Both osmosis and diffusion are essential for maintaining the delicate balance of life within cells and organisms.
Key Concepts
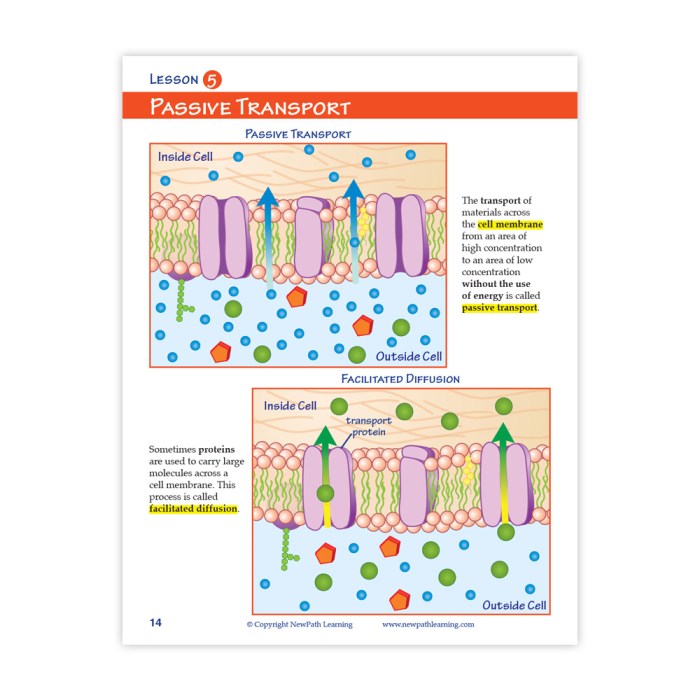
Semipermeable Membranes
Semipermeable membranes are selectively permeable barriers that allow certain substances to pass through while blocking others. They play a crucial role in osmosis and diffusion by controlling the movement of molecules and ions across them.
Concentration Gradients
Concentration gradients refer to the differences in the concentration of a substance across a semipermeable membrane. These gradients drive the movement of molecules and ions, causing osmosis and diffusion.
Factors Affecting Osmosis and Diffusion
Several factors influence the rate of osmosis and diffusion, including:
- Membrane Permeability:The permeability of the membrane to the specific substance affects the rate of movement.
- Concentration Gradient:The greater the concentration gradient, the faster the movement.
- Surface Area:The larger the surface area of the membrane, the faster the movement.
- Temperature:Higher temperatures generally increase the rate of movement.
Types of Osmosis: Quiz On Osmosis And Diffusion
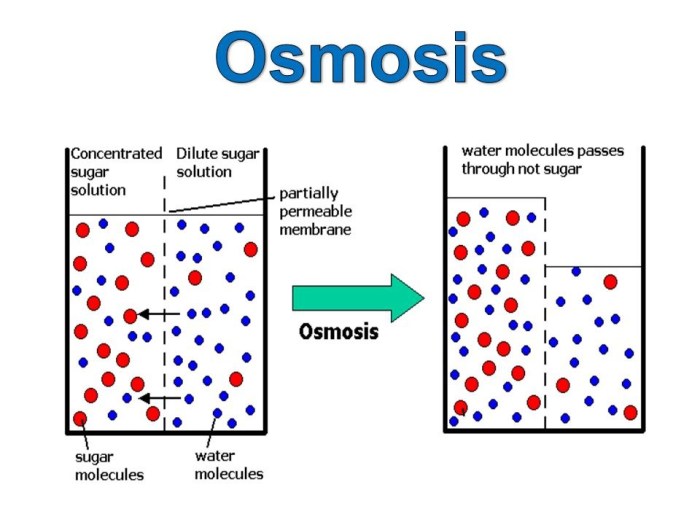
Osmosis is the movement of water across a semipermeable membrane from an area of high water concentration to an area of low water concentration. There are three main types of osmosis: isotonic, hypotonic, and hypertonic solutions.
The type of osmosis that occurs depends on the concentration of solutes in the solution. A solution is isotonic if it has the same concentration of solutes as the cell, hypotonic if it has a lower concentration of solutes than the cell, and hypertonic if it has a higher concentration of solutes than the cell.
Isotonic Solutions
In an isotonic solution, the concentration of solutes inside and outside the cell is the same. This means that there is no net movement of water across the cell membrane. The cell will maintain its normal shape and size.
Hypotonic Solutions, Quiz on osmosis and diffusion
In a hypotonic solution, the concentration of solutes outside the cell is lower than the concentration of solutes inside the cell. This means that water will move into the cell in an attempt to equalize the concentrations. The cell will swell and may eventually burst.
Hypertonic Solutions
In a hypertonic solution, the concentration of solutes outside the cell is higher than the concentration of solutes inside the cell. This means that water will move out of the cell in an attempt to equalize the concentrations. The cell will shrink and may eventually die.
Types of Diffusion
Diffusion is the net movement of particles from an area of high concentration to an area of low concentration. There are two main types of diffusion: simple diffusion and facilitated diffusion.
Simple Diffusion
Simple diffusion is the movement of particles across a semipermeable membrane without the assistance of any carrier proteins. This type of diffusion is driven by the concentration gradient of the particles, and it occurs when the concentration of particles is higher on one side of the membrane than the other.
Small, nonpolar molecules, such as oxygen and carbon dioxide, can easily pass through the lipid bilayer of the cell membrane via simple diffusion.
Facilitated Diffusion
Facilitated diffusion is the movement of particles across a semipermeable membrane with the assistance of carrier proteins. This type of diffusion is also driven by the concentration gradient of the particles, but it requires the presence of a carrier protein that can bind to the particles and transport them across the membrane.
Carrier proteins are specific for the type of particle they transport, and they can increase the rate of diffusion by providing a pathway for the particles to cross the membrane. Glucose and amino acids are examples of molecules that require carrier proteins for facilitated diffusion across the cell membrane.
Applications of Osmosis and Diffusion
Osmosis and diffusion play crucial roles in numerous fields, including medicine, biotechnology, and environmental science. These processes facilitate essential functions in living organisms and have practical applications in various technologies.
In medicine, osmosis is utilized in techniques such as dialysis and osmosis-driven drug delivery systems. Diffusion principles are applied in gas exchange within the lungs and drug absorption through cell membranes.
Take a break from your quiz on osmosis and diffusion to read about the shocking ocean reef golf cart accident . It’s a reminder of the importance of safety and the unexpected events that can occur in our daily lives.
Now, let’s return to our quiz and test your understanding of how substances move across cell membranes.
Biotechnology
- Osmosis is employed in bioreactors to maintain optimal water balance for cell growth and metabolite production.
- Diffusion principles guide the separation of biomolecules, such as proteins and DNA, using techniques like electrophoresis and chromatography.
Environmental Science
- Osmosis regulates water uptake and loss in plants, influencing plant growth and water availability in ecosystems.
- Diffusion governs the movement of pollutants and nutrients in soil and water, affecting environmental health and remediation strategies.
Comparison of Osmosis and Diffusion
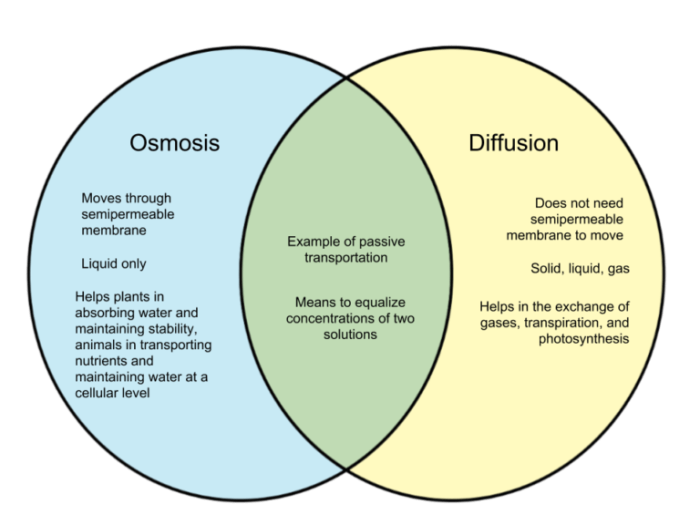
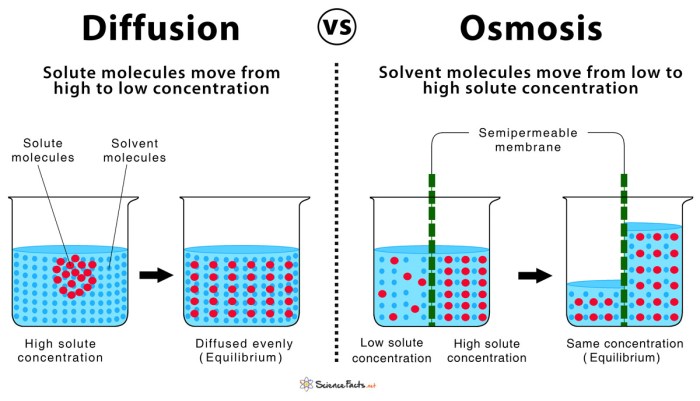
Osmosis and diffusion are both important processes in biology that involve the movement of molecules across a semipermeable membrane. However, there are some key differences between the two processes.
Osmosisis the movement of water molecules across a semipermeable membrane from an area of high water concentration to an area of low water concentration. Diffusionis the movement of molecules from an area of high concentration to an area of low concentration.
The following table summarizes the key differences between osmosis and diffusion:
| Characteristic | Osmosis | Diffusion |
|---|---|---|
| Definition | Movement of water molecules across a semipermeable membrane | Movement of molecules from an area of high concentration to an area of low concentration |
| Direction of movement | From an area of high water concentration to an area of low water concentration | From an area of high concentration to an area of low concentration |
| Effect on cells | Can cause cells to swell or shrink | Can cause molecules to move into or out of cells |
Illustrative Examples
To fully grasp the concepts of osmosis and diffusion, let’s delve into some illustrative examples that vividly demonstrate these processes.
Imagine a simple experiment involving two compartments separated by a selectively permeable membrane. One compartment contains a concentrated sugar solution, while the other holds pure water. When the membrane is introduced, a remarkable phenomenon occurs.
Diffusion
Water molecules, being smaller and more mobile, move across the membrane from the compartment with lower sugar concentration (pure water) to the compartment with higher sugar concentration (sugar solution). This movement continues until the sugar concentration is equalized across both compartments, establishing a state of equilibrium.
Osmosis
Now, let’s modify the experiment slightly. Instead of pure water in one compartment, we introduce a dilute sugar solution. As the membrane is permeable to water but not to sugar molecules, water molecules move from the dilute sugar solution (lower sugar concentration) to the concentrated sugar solution (higher sugar concentration) to equalize the water concentration.
FAQ Guide
What is the difference between osmosis and diffusion?
Osmosis is the movement of water across a semipermeable membrane from an area of high water concentration to an area of low water concentration. Diffusion, on the other hand, is the movement of particles from an area of high concentration to an area of low concentration, regardless of the presence of a membrane.
What is the role of semipermeable membranes in osmosis and diffusion?
Semipermeable membranes allow the passage of certain molecules while blocking others. In osmosis, water molecules can pass through the membrane, while solute molecules cannot. In diffusion, small molecules can pass through the membrane, while larger molecules cannot.
How do concentration gradients affect osmosis and diffusion?
Concentration gradients provide the driving force for both osmosis and diffusion. Water molecules move from areas of high water concentration to areas of low water concentration, while particles move from areas of high concentration to areas of low concentration.